
The most common example of an ionic compound is table salt(NaCl). Ionic Compound:Ĭompounds that consist of ions joined together by electrostatic forces are called ionic compounds. Na+ & Cl- ions) can stick or bind together by the electrostatic force of attraction known as an ionic bond. What is the most accepted definition of an ionic bond vs polar covalent vs. so the ions having opposite charges( e.g. We know that opposite charges always attract one another. For example, Chloride ion (Cl –) is an anion.

Whereas Negatively charged ions are formed by the gain of electron/s and are known as Anion. The charge distributions of the sodium and chloride ions are spherically symmetric, and the chloride ion is about two times the diameter of the sodium ion. Ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions by combination of ions with other particles or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the formerly. Positively charged ions are called cations negatively charged ions, anions. The sodium and chloride ions are arranged in a face-centered cubic (FCC) structure. ion, any atom or group of atoms that bears one or more positive or negative electrical charges. For example, sodium ion (Na+) is a cation. 2: Structure of the sodium chloride crystal. Covalent bonding: Formed between two atoms of non-metals. Positively charged ions are formed by the loss of electron/s and are known as a cation. Ionic bonding: Formed between many ions formed by metal and nonmetallic elements. When atoms get an electrical charge they are called ions. When Cl-atom gains one electron from Na-atom, Cl-atom becomes a chloride ion (Cl –).A chloride ion is formed after the gain of one electron and is left with a complete octet ( 8 valence shell electrons). To complete its valence shell and becomes stable chlorine needs one electron. Atoms linked together in this way are called ionic compounds. On the other hand, chlorine belongs to group 7A, having 7 valence shell electrons. An ionic bond occurs when one atom gives an electron to another atom. When the Na-atom loses it valence shell electron results in the formation of sodium ion (Na +). To complete its valence shell and becomes stable, sodium atom loses its one valence shell electron and is left with an octet Sodium (Na) belongs to group 1A in the periodic table. Sodium Chloride (NaCl) is formed when two types of atoms (metal & non-metal atoms) stick or bind together by an ionic bond. Covalent bonding involves the sharing of electrons between two.
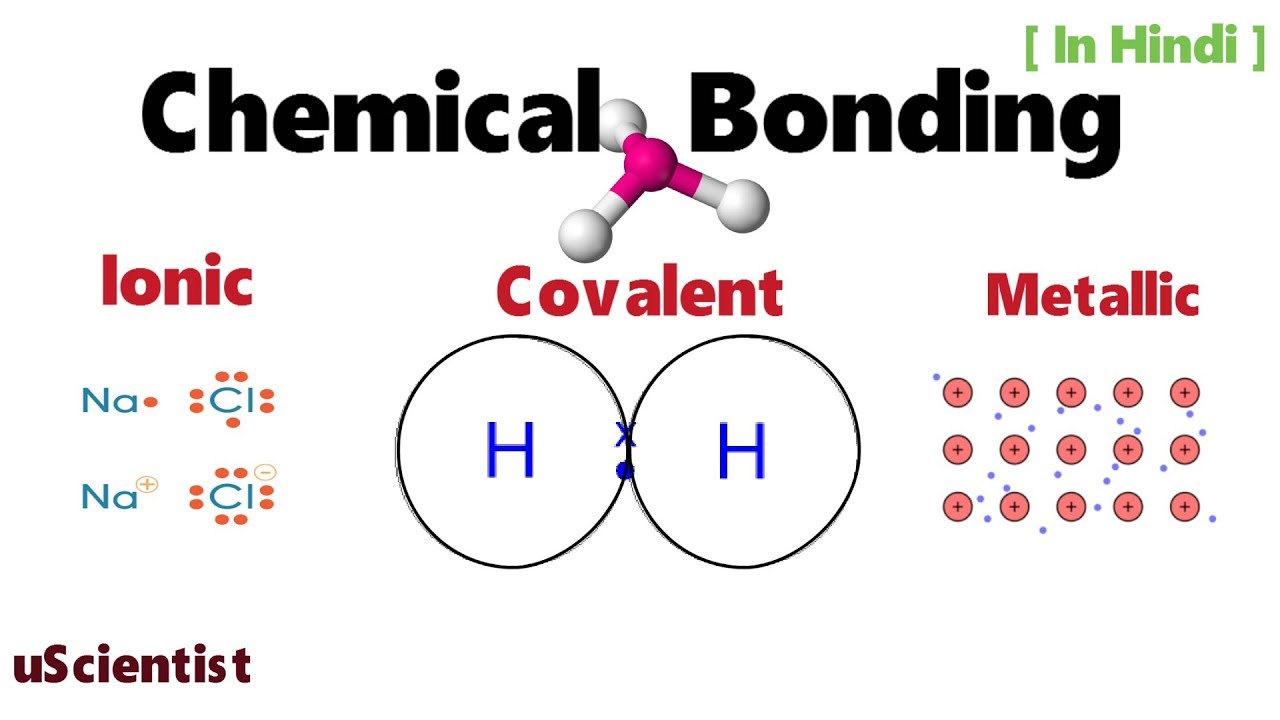
NaCl is the salt (also known as table salt) that we add to our foods to give flavour. There are primarily two forms of bonding that an atom can participate in: Covalent and Ionic. To understand ionic bonding, let’s take an example of sodium chloride (NaCl). Diagram of Ionic Bonding: Ionic Bond Example: The complete transfer of electron/s from one atom(metal) to another atom (non-metal) results in the formation of an ionic bond. The force of attraction that binds oppositely charged ions is called an ionic bond Such a type of bond forms when one atom completely transfer valence (outermost) shell electron/s to another atom. An ionic bond is also known as the electrovalent bond is the type of chemical bond where a linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.


 0 kommentar(er)
0 kommentar(er)
